Features
- MiSight® 1 day covers nearly 100% of spherical prescriptions for age-appropriate children with myopia1°
- Easy to fit, single use lens
- Corrects refractive error and slows the elongation of the eye through ActivControlTM Technology
- Suitable for age-appropriate children*
The MiSight® 1 day lens is clinically proven to slow the progression of myopia in age-appropriate children.2*
The efficacy of MiSight 1 day was validated in a multi-year clinical study that enrolled children between 8 and 12 years old. The MiSight® 1 day clinical study is the longest continuous soft contact lens study for myopia management.
Clinical Study - Part 1
Over three years, MiSight® 1 day reduced the rate of myopia progression by 59% on average, compared to a single vision 1-day lens.2
Change in Spherical Equivalent Refractive Error (SERE)
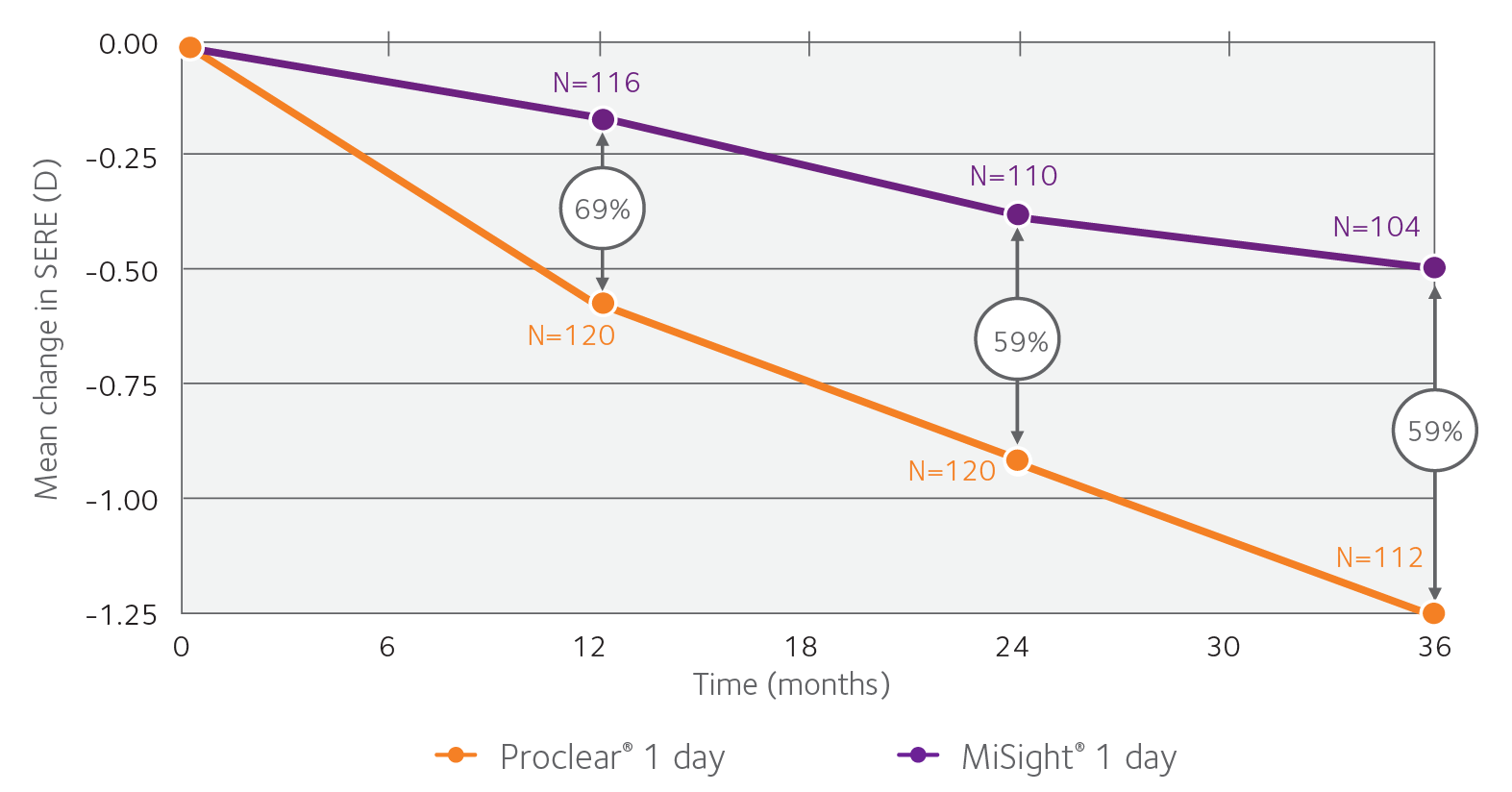
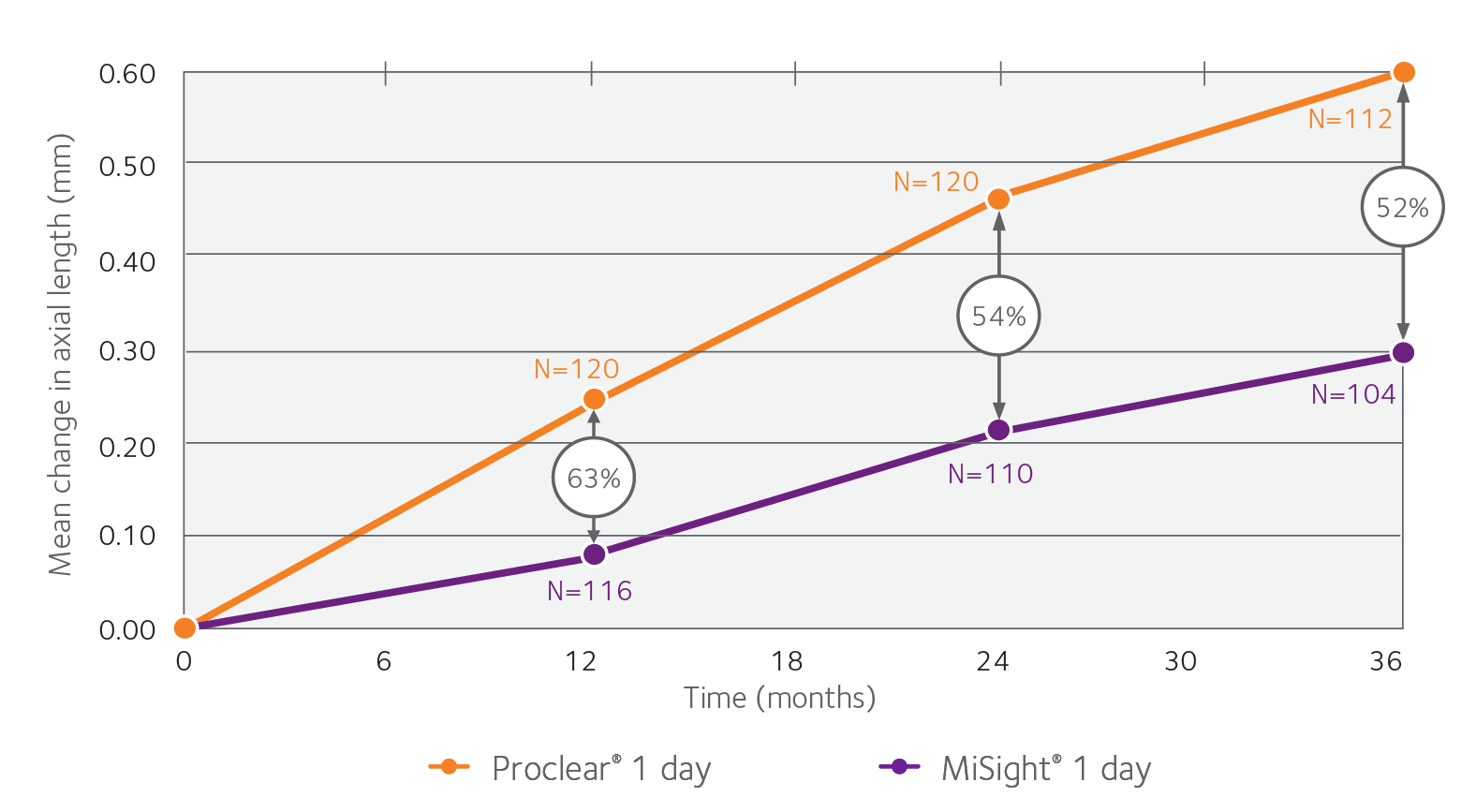
With MiSight 1 day, over a three year period, there was also a 52% average reduction in axial lengthening, compared to a single vision 1-day lens.2
Change in axial length
Clinical Study - Part 2
In Part 2 (years 4 to 6) of the MiSight® 1 day clinical study, children in the control group wearing Proclear® 1 day were switched into MiSight® 1 day.
New and established MiSight® 1 day wearers had comparable rates of myopic progression and axial length growth.3 Children†† adapted to spherical contact lenses achieved excellent visual acuity‡ when they switched to MiSight® 1 day.3
The clinical study of MiSight® 1 day lenses was the first to demonstrate sustained slowing of eye growth with a soft contact lens over time.4†
Clinical Study - Part 3
In the seventh year of the MiSight® 1 day clinical study, treatment was discontinued for all participants. Data indicated that there was no rebound effect with MiSight® 1 day contact lenses.5,6±
Peer-Reviewed Papers
The MiSight® 1 day clinical study findings have been published in Optometry and Vision Science, the peer review journal of the American Academy of Optometry.
The peer-reviewed paper entitled, Long-Term Effect of Dual-Focus Contact Lenses on Myopia Progression in Children: A 6-year Multicenter Clinical Trial (Chamberlain P, et al.) is now available.
This new paper joins a growing body of peer-reviewed research stemming from the CooperVision MiSight® 1 day study including Ocular Health of Children Wearing Daily Disposable Contact Lenses Over a 6-year Period (Woods J, et al.) and Axial Length Targets for Myopia Control (Chamberlain P, et al.), as well as the recent Garland W. Clay Award winner, A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control (Chamberlain P, et al.).
How MiSight 1 day works
MiSight 1 day with ActivControl™ Technology helps slow the elongation of the eye and reduces myopia progression, while fully correcting refractive error.2 Addressing axial elongation helps to reduce the risk of myopia-related vision complications later in life, including irreversible vision loss.7
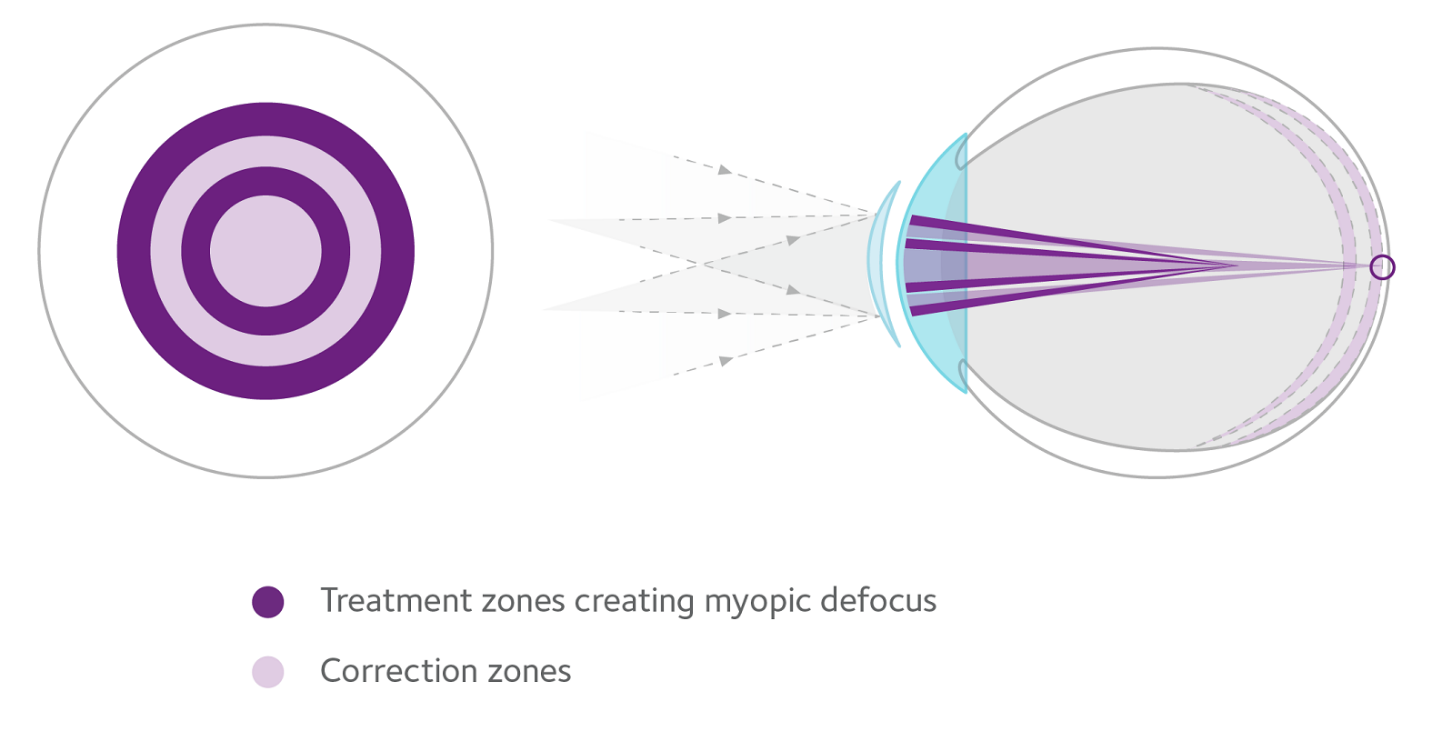
- Two treatment zones create myopic defocus with image focus in front of the retina, rather than behind it to slow axial elongation
- Two correction zones correct myopia in all gaze positions
Are you interested in MiSight® 1 day, but are not yet certified? Let’s get you started.
Certified Eye Care Professionals have access to the Brilliant Futures™ program, including marketing assets to support you, your practice and staff, myopic pediatric patients and their parents. Check out MiSightPro to see what is available to you.
Certified Eye Care Professionals can also consistently stay connected with the parents of MiSight® 1 day patient in between follow-up appointments. Sign up parents to receive relevant, timely messages, regardless of where their children are in their myopia management journey.
Product Details
References:
* Canadian Indications for Use: MiSight (omafilcon A) Soft Contact Lenses for Myopia Control may reduce the rate of myopia progression in children (6-18) and correct ametropia. Reduction of myopia progression was observed in children with wearing time of 12 hours (8-16 hours) per day, 6.4 days (5-7) per week in a clinical study. Permanent myopia control after lens treatment is discontinued is not supported by clinical studies. MiSight (omafilcon A) Soft Contact Lenses for Myopia Control are indicated for single use daily disposable wear. When prescribed for daily disposable wear, the lens is to be discarded after each removal.
° Includes prescriptions up to 0.75DC.
†† Median age at switching 13.0 ± 1.5 years.
‡ VA (LogMAR) > 6/6 (20/20) at all visits from dispensing to 6-year visit.
† While eyes are still growing; children fit ages 8-12 and followed for 6-years. n=40
± On average, for children aged 8-15 at initiation of treatment, there was no indication that accumulated treatment effect gained following 3 or 6 years of MiSight® 1 day wear was lost during a 12-month cessation study. Instead, eye growth reverted to expected, age average myopic progression rates.
CVI Data on file, 2022. SERE coverage of childhood myopia prescriptions with MiSight® 1 day for 104,810 eyes in Asia (China, Korea) and 116,336 eyes in Europe and USA aged 8-18 years.
Chamberlain P et al A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci 2019;96:556-567
Chamberlain P, Arumugam B, Jones D et al. Myopia Progression in Children wearing Dual-Focus Contact Lenses: 6-year findings. Optom Vis Sci 2020;97(E-abstract): 200038
Chamberlain P et al. Long-Term Effect of Dual-Focus Contact Lenses on Myopia Progression in Children: A 6-year Multicenter Clinical Trial. Optom Vis Sci 2022 In Press.
Chamberlain P, Arumugam B, et al. Myopia progression on cessation of Dual-Focus contact lens wear: MiSight 1 day 7 year findings. Optom Vis Sci 2021;98:E-abstract 210049.
Hammond D, Arumugam B, et al. Myopia Control Treatment Gains are Retained after Termination of Dual-focus Contact Lens Wear with no Evidence of a Rebound Effect. Optom Vis Sci 2021;98:E-abstract 215130.
Tideman JW, et al. Association of Axial Length With Risk of Uncorrectable Visual Impairment for Europeans With Myopia. JAMA Ophthalmol. 2016;134(12):1355-63.







